Initiation By Diacyl Peroxides
Free radicals can be generated by a number of compounds, called initiators.1 One of the most important classes of initiators are diacyl peroxides, which have the general formula R1C(O)OOC(O)R2, wherein R1 and R2 represent alkyl and/or aryl groups. All diacyl peroxides are thermally unstable and decompose at relative low temperatures thereby generating free radicals.
The most important indicator for the activity of an initiator is its half-life, t1/2, which is the time required for half of the initial initiator to decompose at a given temperature. In the case of first order decomposition kinetics, which is true for most initiators, the half-life is given by
t1/2 = ln 2/kd
Benzoyl Peroxide
The by far most important organic peroxide initiator is benzoyl peroxide (BPO). It consists of two benzoyl groups bridged by a peroxide link. BPO readily undergoes symmetrical fission (homolysis), forming two benzoyloxy radicals:2-4
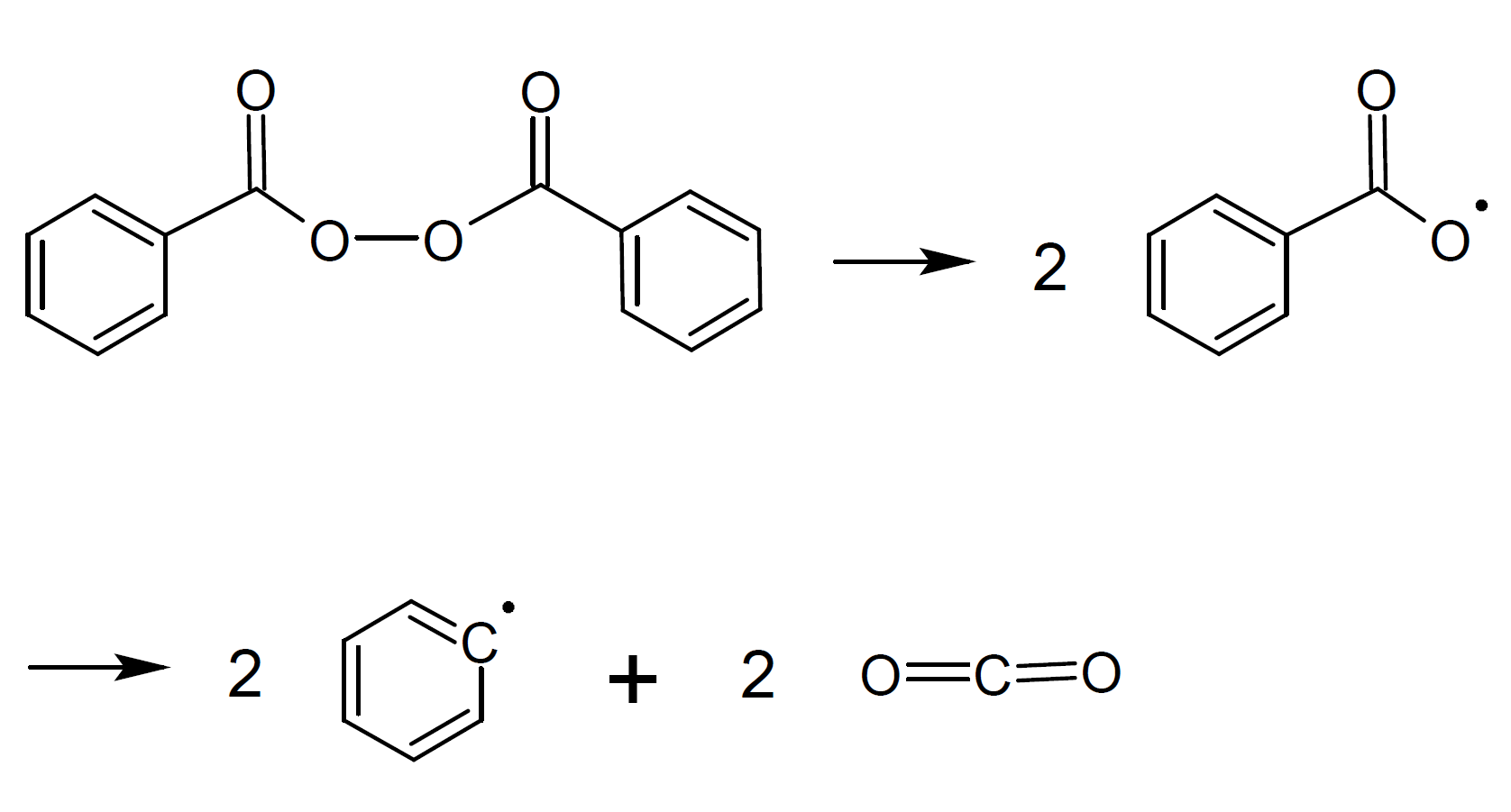
The two fragments with unpaired electrons are called free radical initiators. Following its generation, the initiating free radicals react with a monomer unit thereby creating growing polymer chains.
The reactivity of BPO depends on the solvent and monomer. In the absence of accelerators, the decomposition rate of diacyl peroxides is of first order, that is, it is directly proportional to the initiator concentration:4,5
RI = d[I·] / dt = -2 d[I] / dt = 2 f kd [I]
which after integration gives
[I] = [I]0 · exp[- f kd t]
where f is the efficiency of initiation defined as4
f = rate of initiation of polymerization / 2 x rate of decomposition of initiator
As a rough rule, the rate of decomposition of peroxides in various solvents decreases in the order: highly halogenated aliphatics < aromatics < most aliphatics < ethers and alcohols < amines. The effect of substituents in either or both of the aromatic rings has been investigated extensively. The rate of decomposition increases by electron donating groups and decreases by electron withdrawing groups as one might expect, because an increase in electron density on the oxygen atoms increases repulsion between them and therefore enhances the rate of spontaneous cleavage of the O-O bond.
The decomposition of benzoyl peroxide is considerably accelerated by the presence of amines (reducing agent). Particularly effective are tertiary aromatic amines such as dimethylanilne which accelerate peroxide decomposition by one-electron transfer:6,7
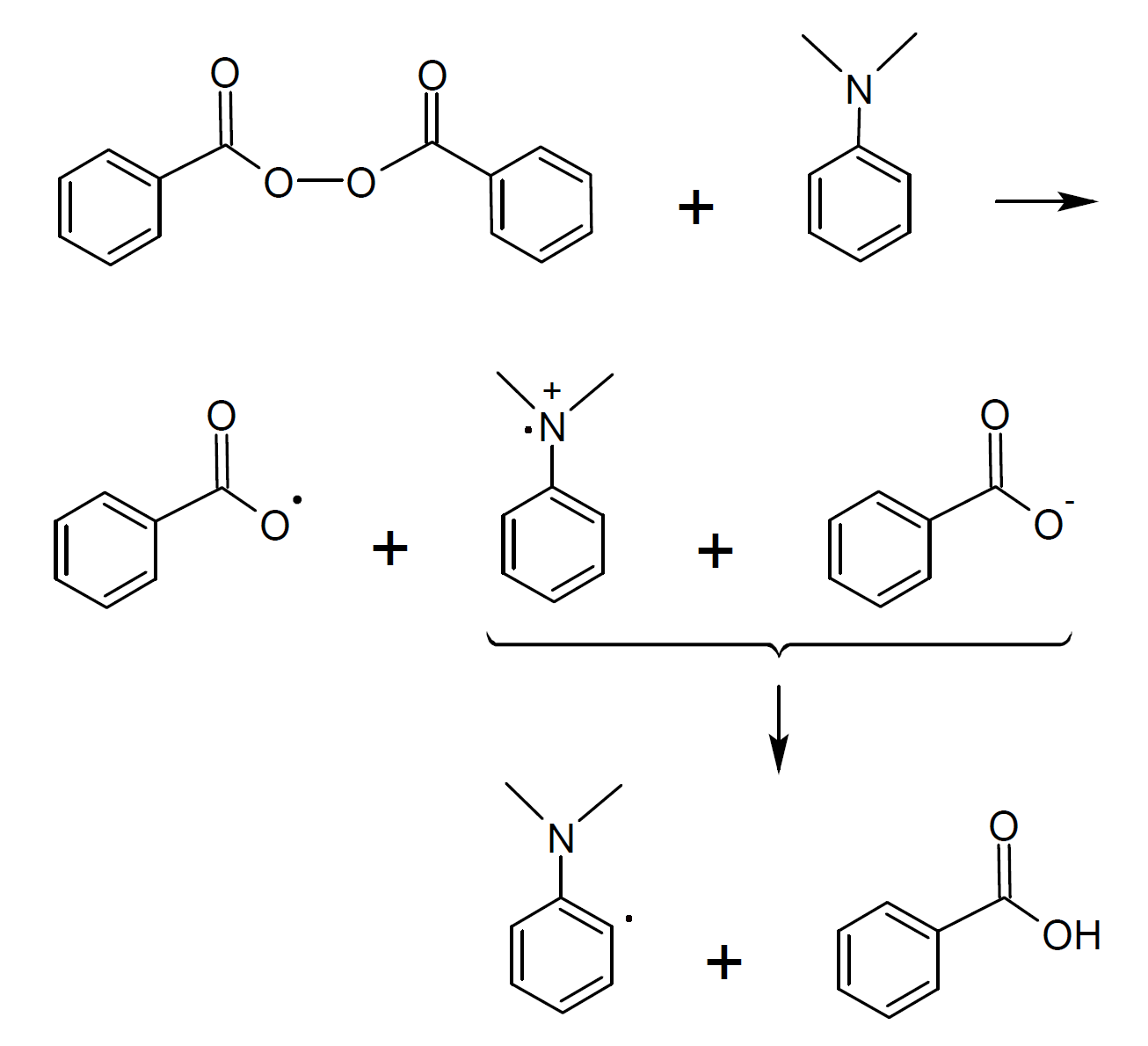
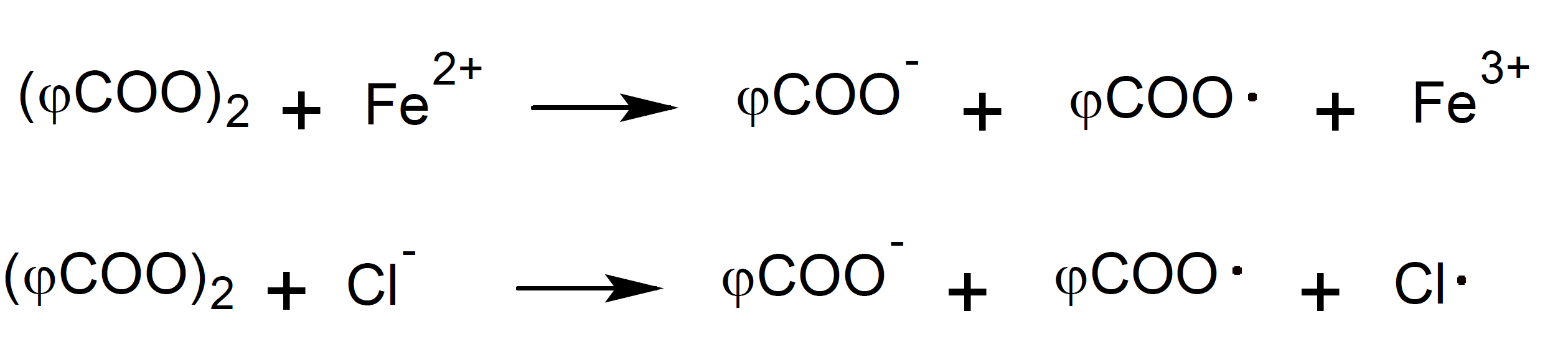
A similar one-electron transfer is observed when chloride ions (Cl-) or ferrous ions (Fe2+) react with peroxides:6
Di-t-butyl Peroxide
Di-tert-butyl peroxide (DTBP) is another important free radical initiator which is mainly used for the initiation of LDPE polymerization and for cross-linking elastomers such as silicone and EPDM. It is special as an initiator because its thermal decomposition temperature is above 100 °C and, thus, it is much higher than that of most other initiators. Although there are several other initiators with a high decomposition temperature, such as aliphatic dialkyl peroxides, DTBP is often preferred because it is easier to handle due to its lower explosion hazard. It can also be easily purified.
DTBP undergoes symmetrical fission (homolysis), forming two t-butoxy radicals. This reaction is followed by a competiton between disproportionation (dismutation) and hydrogen abstraction from another molecule, which can be a solvent molecule or peroxide itself. Disproportionation yields acetone and a methyl radical whereas hydrogen abstraction leads to t-butyl alcohol and, in somce cases, to iso-butylene oxide, and a t-butoxy radical:6,8
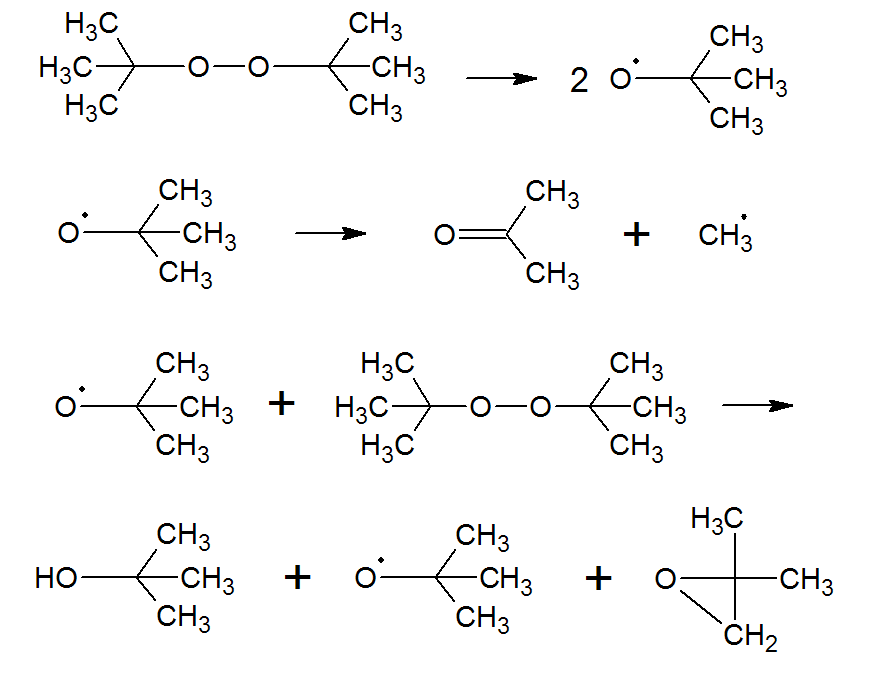
Two other common diacyl peroxides are dilauroyl and didecanoyl peroxide which have an even shorter half-life than dibenzoyl peroxide.
References & Notes
The initiators are sometimes erroneously called catalysts. Initiators, however, are consumed in the reaction while catalysts are regenerated after the completion of the reaction.
- J. C. Bevington and J. Toole, J. Poly. Sci., 28, 413 - 420 (1958)
- A. Ravve, Principles of Polymer Chemistry, 3rd Edition 2012
- Paul J. Flory, Principles of Polymer Chemistry, 1st Edition 1953
Some of the benzyl radicals and benzoyloxy radicals will recombine which reduces the efficiency of initiation.
C. H. Bamford, W. G. Barb, A. D. Jenkins, and P. F. Onyon, The Kinetics of Vinyl Polymerization by Radical Mechanism, London 1958
- T. T. Vasil'eva, and R. Kh. Freidlina, Russ. Chem. Bull., 17, 1039 - 1041 (1968)
- J. Kruzelak, R. Sykora, and I. Hudec, J. Poly. Eng., 34, 617 - 624 (2014)
Revised October 8, 2019